

Corporate Overview April 2020 EXHIBIT 99.2

This presentation contains forward-looking statements for the purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995 and other federal securities laws. The use of words such as “may,” “might,” “will,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “intend,” “future,” “potential,” “continue” and other similar expressions (as well as other words or expressions referencing future events, conditions, or circumstances) are intended to identify forward-looking statements. For example, statements Immunovant makes regarding our business strategy, our plans to develop and commercialize our product candidates, the potential safety and efficacy of our product candidates, our expectations regarding timing, the design and results of clinical trials of our product candidates, our plans and expected timing with respect to regulatory filings and approvals, the size and growth potential of the markets for our product candidates, and our ability to serve those markets, and our plans and expected timing with respect to regulatory filings and approvals are forward-looking. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. The product candidates that Immunovant develops may not progress through clinical development or receive required regulatory approvals within expected timelines or at all. In addition, promising interim results or other preliminary analyses do not in any way ensure that later or final results in a clinical trial or in related or similar clinical trials will replicate those interim results. In addition, clinical trials may not confirm any safety, potency or other product characteristics described or assumed in this press release. In addition, such product candidates may not be beneficial to patients, or even if approved by regulatory authorities, successfully commercialized. The failure to meet expectations with respect to any of the foregoing matters may have a negative effect on Immunovant’s stock price. All forward-looking statements are based on estimates and assumptions by Immunovant’s management that, although Immunovant believes to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that Immunovant expected. Such risks and uncertainties include, among others, the initiation and conduct of preclinical studies and clinical trials; the availability of data from clinical trials; the expectations for regulatory submissions and approvals; the continued development of Immunovant’s product candidates and platforms; Immunovant’s scientific approach and general development progress; Immunovant’s ability to maintain or scale up manufacturing processes and transition such processes; the availability and commercial potential of Immunovant’s product candidates including the size of potentially addressable markets and degree of market acceptance; and the potential impact of the recent COVID-19 pandemic on Immunovant’s clinical development plans and timelines. These statements are also subject to a number of material risks and uncertainties that are described in Immunovant’s periodic and other reports filed with the Securities and Exchange Commission (SEC), including the risk factors detailed in Immunovant’s most recent Quarterly Report on Form 10-Q filed with the SEC on February 14, 2020. Any forward-looking statement speaks only as of the date on which it was made. Immunovant undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise.

Immunovant
Our vision: Normal lives for patients with autoimmune diseases Our asset: IMVT-1401, a novel, fully human monoclonal antibody inhibiting FcRn-mediated recycling of IgG Our strategy for IMVT-1401: Be best-in-class in target indications where anti-FcRn mechanism has already established clinical proof-of-concept Be first to study FcRn inhibition in target indications with clear biologic rationale and no known in-class competition Our near-term value drivers: Four anticipated data readouts over the next 20 months
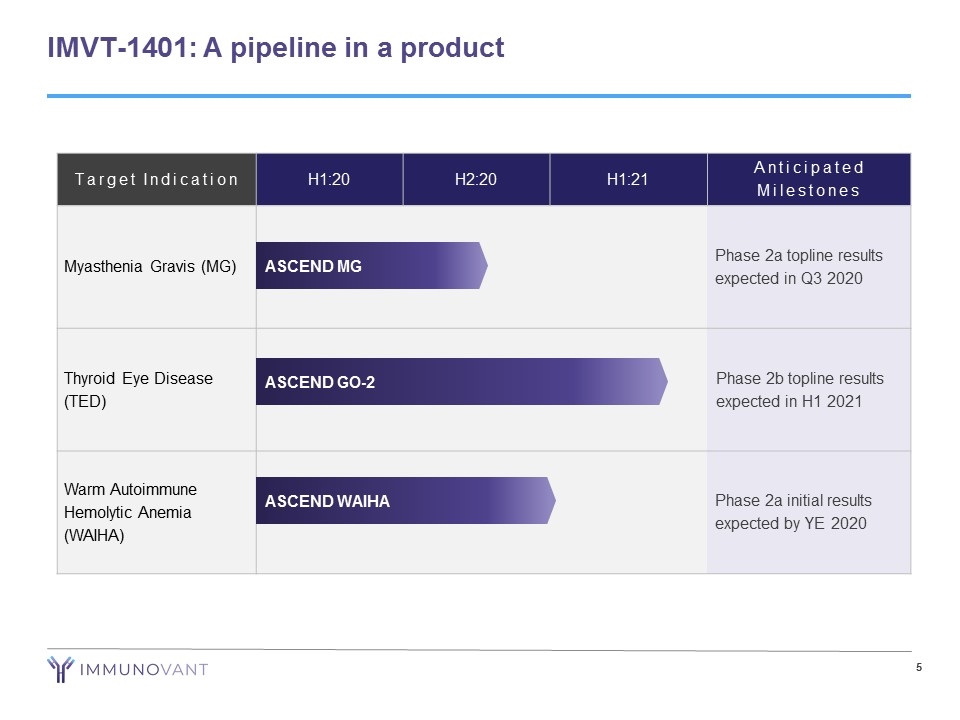
IMVT-1401: A pipeline in a product Target Indication H1:20 H2:20 H1:21 Anticipated Milestones Myasthenia Gravis (MG) Phase 2a topline results expected in Q3 2020 Thyroid Eye Disease (TED) Phase 2b topline results expected in H1 2021 Warm Autoimmune Hemolytic Anemia (WAIHA) Phase 2a initial results expected by YE 2020 ASCEND MG ASCEND GO-2 ASCEND WAIHA
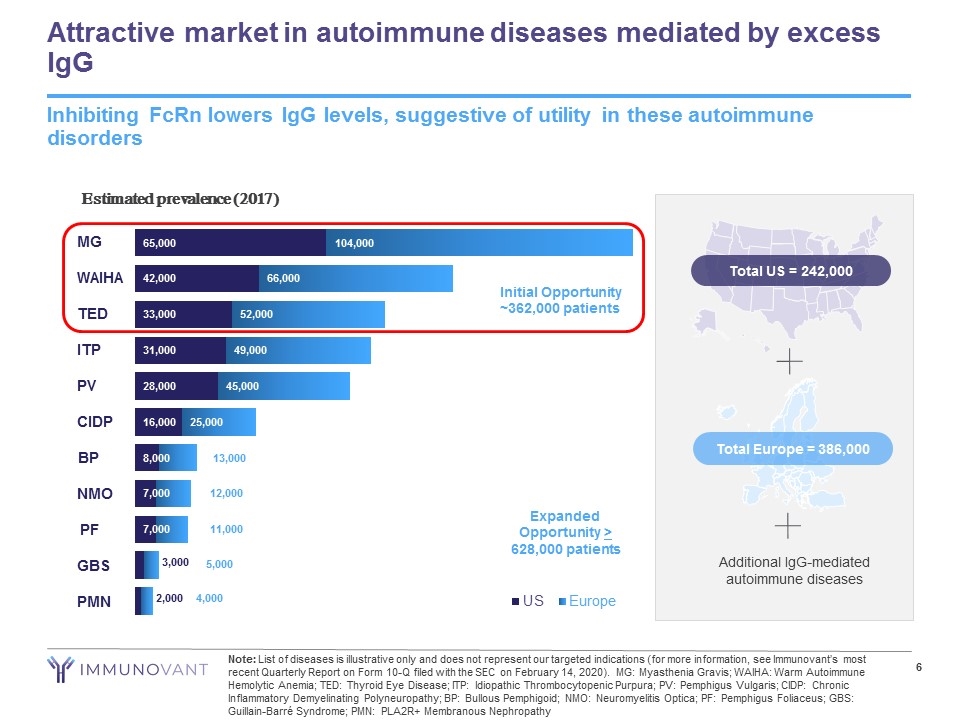
Attractive market in autoimmune diseases mediated by excess IgG Inhibiting FcRn lowers IgG levels, suggestive of utility in these autoimmune disorders Note: List of diseases is illustrative only and does not represent our targeted indications (for more information, see Immunovant’s most recent Quarterly Report on Form 10-Q filed with the SEC on February 14, 2020). MG: Myasthenia Gravis; WAIHA: Warm Autoimmune Hemolytic Anemia; TED: Thyroid Eye Disease; ITP: Idiopathic Thrombocytopenic Purpura; PV: Pemphigus Vulgaris; CIDP: Chronic Inflammatory Demyelinating Polyneuropathy; BP: Bullous Pemphigoid; NMO: Neuromyelitis Optica; PF: Pemphigus Foliaceus; GBS: Guillain-Barré Syndrome; PMN: PLA2R+ Membranous Nephropathy Additional IgG-mediated autoimmune diseases Total Europe = 386,000 Total US = 242,000 Initial Opportunity ~362,000 patients Estimated prevalence (2017) Expanded Opportunity > 628,000 patients MG WAIHA TED ITP PV CIDP BP NMO PF GBS PMN

IMVT-1401
IgG antibodies implicated in certain autoimmune diseases Antibodies play an important role in immune defense against pathogens1 Clearing bacteria, viruses, and other harmful organisms and substances Eliciting an immune response that leads to inflammation IgG antibody subclass accounts for ~75% of antibodies in the plasma of healthy people1 In many autoimmune diseases, IgG antibodies develop that can recognize and bind to normal tissues2 Targets may include cell-surface receptors or circulating proteins Result is a harmful immune response that damages critical tissues and organs Predisposing factors may include genetic susceptibility, environmental triggers, and factors not yet known3 IgG IgE IgM IgA IgD Antibodies in healthy individuals Antibodies in autoimmune disease Leusen J.H.W. The Role of IgG in Immune Responses. Molecular and Cellular Mechanisms of Antibody Activity, 2013 Isabela S., et al. The role of autoantibodies in health and disease. Romanian Journal of Morphology and Embryology, 2016 Mariani S.M. Genes and autoimmune diseases - a complex inheritance. MedGenMed, 2004
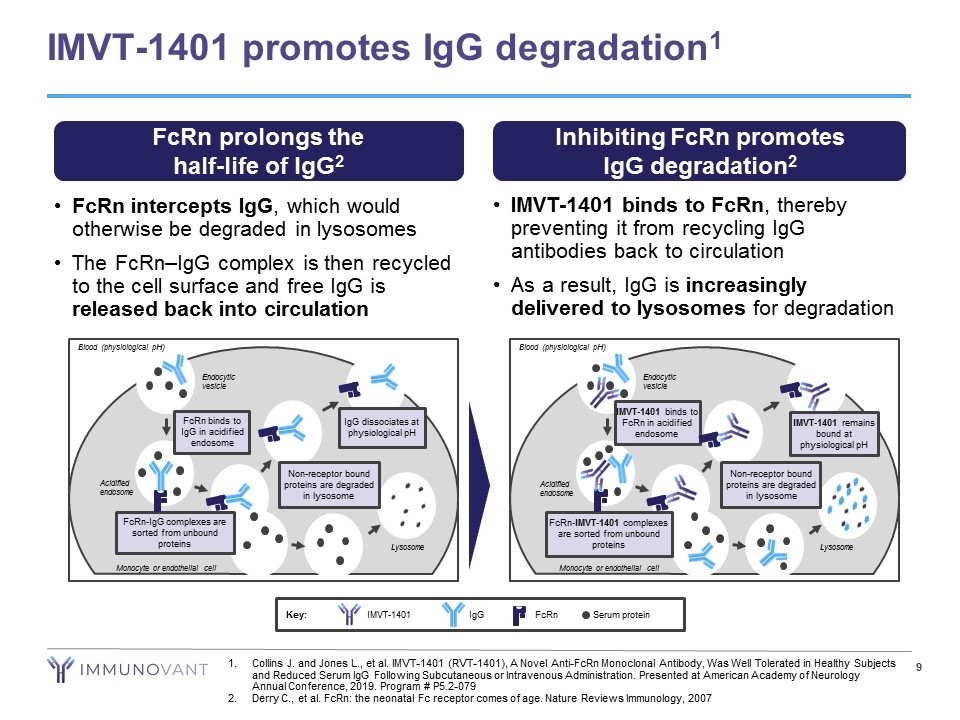
FcRn intercepts IgG, which would otherwise be degraded in lysosomes The FcRn–IgG complex is then recycled to the cell surface and free IgG is released back into circulation IMVT-1401 binds to FcRn, thereby preventing it from recycling IgG antibodies back to circulation As a result, IgG is increasingly delivered to lysosomes for degradation IMVT-1401 promotes IgG degradation1 FcRn prolongs the half-life of IgG2 Inhibiting FcRn promotes IgG degradation2 Blood (physiological pH) Endocytic vesicle Acidified endosome FcRn-IgG complexes are sorted from unbound proteins FcRn binds to IgG in acidified endosome Non-receptor bound proteins are degraded in lysosome IgG dissociates at physiological pH Lysosome Monocyte or endothelial cell Blood (physiological pH) Endocytic vesicle Acidified endosome FcRn-IMVT-1401 complexes are sorted from unbound proteins IMVT-1401 binds to FcRn in acidified endosome Non-receptor bound proteins are degraded in lysosome Lysosome Monocyte or endothelial cell IMVT-1401 remains bound at physiological pH Key: Serum protein IgG IMVT-1401 FcRn Collins J. and Jones L., et al. IMVT-1401 (RVT-1401), A Novel Anti-FcRn Monoclonal Antibody, Was Well Tolerated in Healthy Subjects and Reduced Serum IgG Following Subcutaneous or Intravenous Administration. Presented at American Academy of Neurology Annual Conference, 2019. Program # P5.2-079 Derry C., et al. FcRn: the neonatal Fc receptor comes of age. Nature Reviews Immunology, 2007
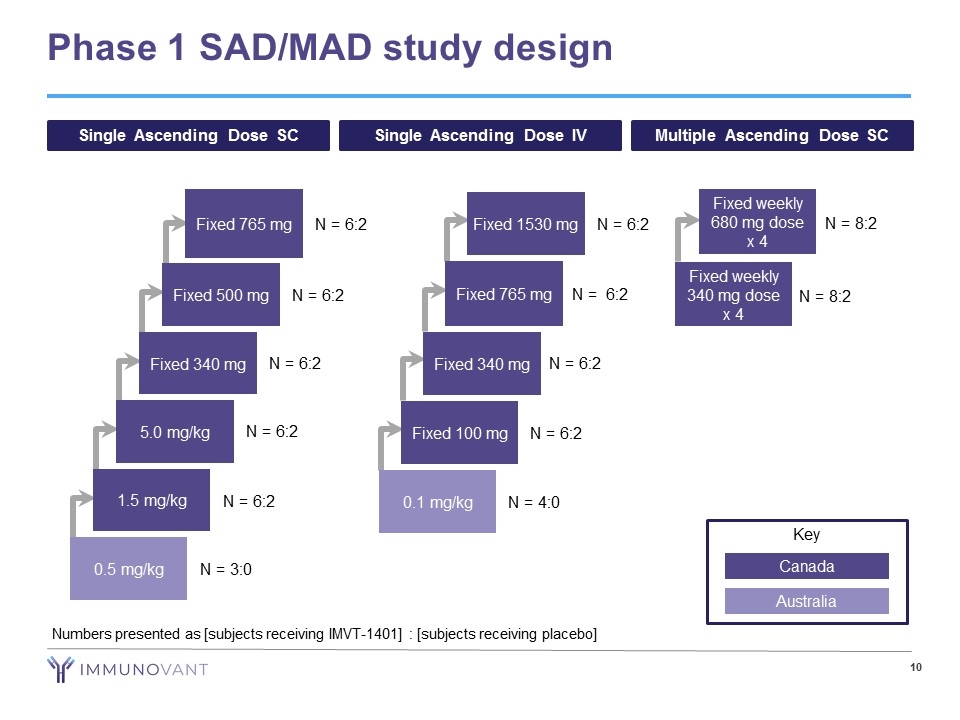
Phase 1 SAD/MAD study design Single Ascending Dose SC Single Ascending Dose IV Multiple Ascending Dose SC 0.5 mg/kg N = 3:0 1.5 mg/kg N = 6:2 5.0 mg/kg N = 6:2 Fixed 340 mg N = 6:2 Fixed 500 mg N = 6:2 Fixed 765 mg N = 6:2 Fixed 100 mg N = 6:2 Fixed 340 mg N = 6:2 Fixed 765 mg N = 6:2 Fixed 1530 mg N = 6:2 0.1 mg/kg N = 4:0 Fixed weekly 680 mg dose x 4 N = 8:2 Fixed weekly 340 mg dose x 4 N = 8:2 Key Canada Australia Numbers presented as [subjects receiving IMVT-1401] : [subjects receiving placebo]
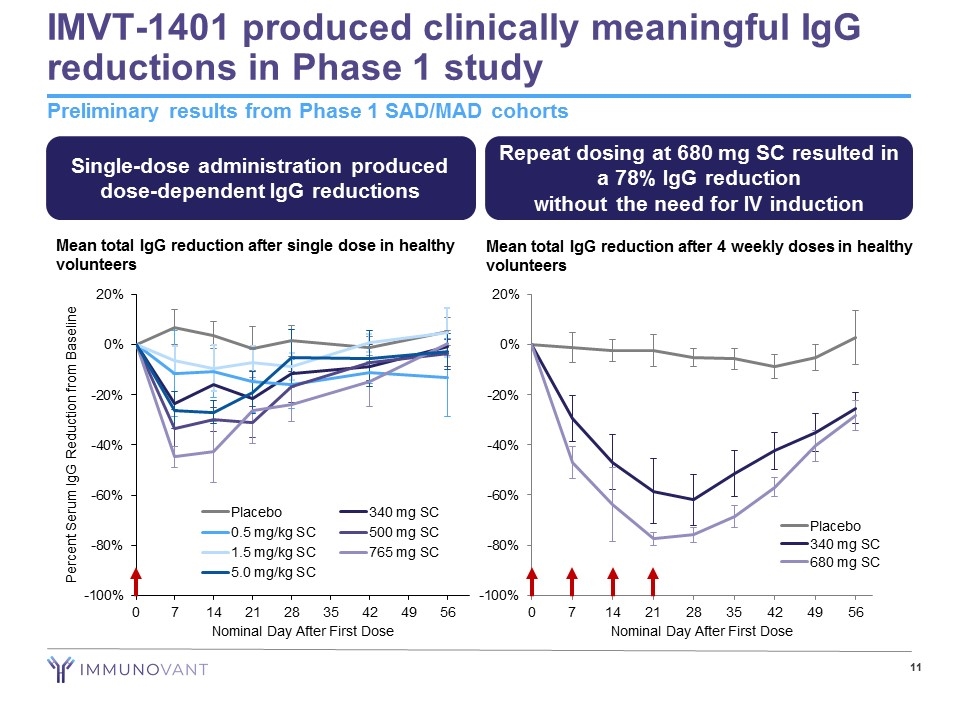
IMVT-1401 produced clinically meaningful IgG reductions in Phase 1 study Preliminary results from Phase 1 SAD/MAD cohorts Mean total IgG reduction after single dose in healthy volunteers Mean total IgG reduction after 4 weekly doses in healthy volunteers Single-dose administration produced dose-dependent IgG reductions Repeat dosing at 680 mg SC resulted in a 78% IgG reduction without the need for IV induction
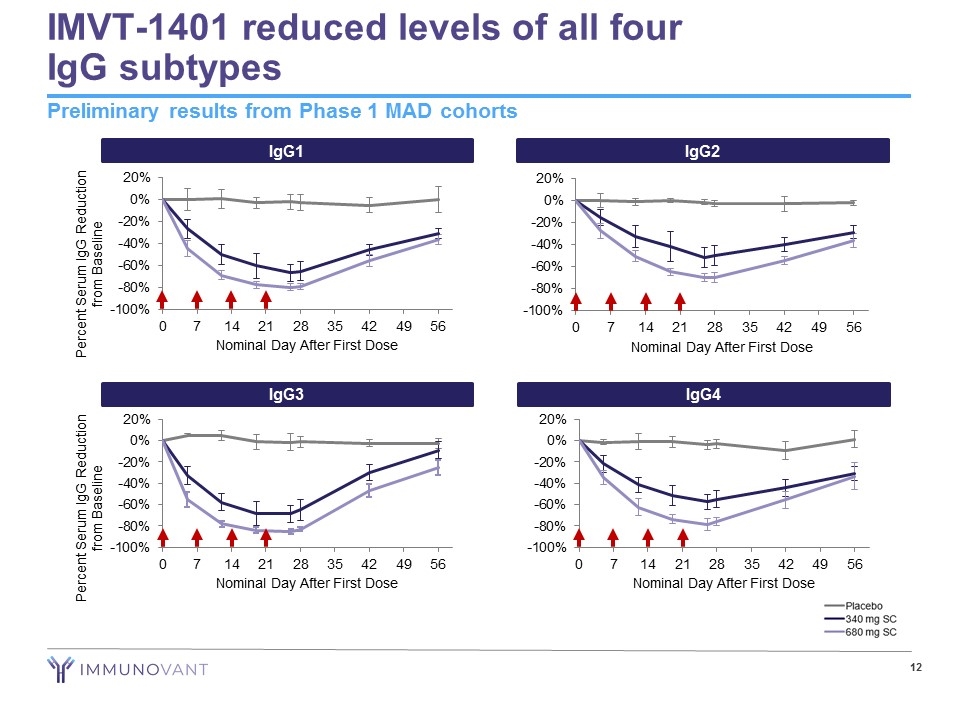
IMVT-1401 reduced levels of all four IgG subtypes Preliminary results from Phase 1 MAD cohorts IgG2 IgG4 IgG1 Percent Serum IgG Reduction from Baseline IgG3 Percent Serum IgG Reduction from Baseline
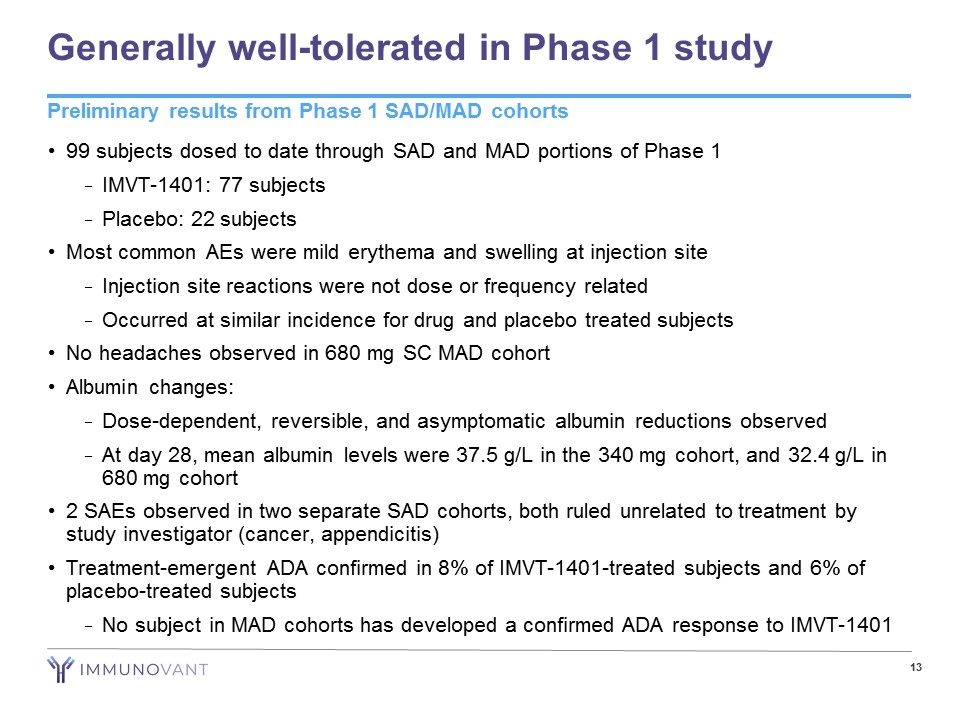
Generally well-tolerated in Phase 1 study 99 subjects dosed to date through SAD and MAD portions of Phase 1 IMVT-1401: 77 subjects Placebo: 22 subjects Most common AEs were mild erythema and swelling at injection site Injection site reactions were not dose or frequency related Occurred at similar incidence for drug and placebo treated subjects No headaches observed in 680 mg SC MAD cohort Albumin changes: Dose-dependent, reversible, and asymptomatic albumin reductions observed At day 28, mean albumin levels were 37.5 g/L in the 340 mg cohort, and 32.4 g/L in 680 mg cohort 2 SAEs observed in two separate SAD cohorts, both ruled unrelated to treatment by study investigator (cancer, appendicitis) Treatment-emergent ADA confirmed in 8% of IMVT-1401-treated subjects and 6% of placebo-treated subjects No subject in MAD cohorts has developed a confirmed ADA response to IMVT-1401 Preliminary results from Phase 1 SAD/MAD cohorts
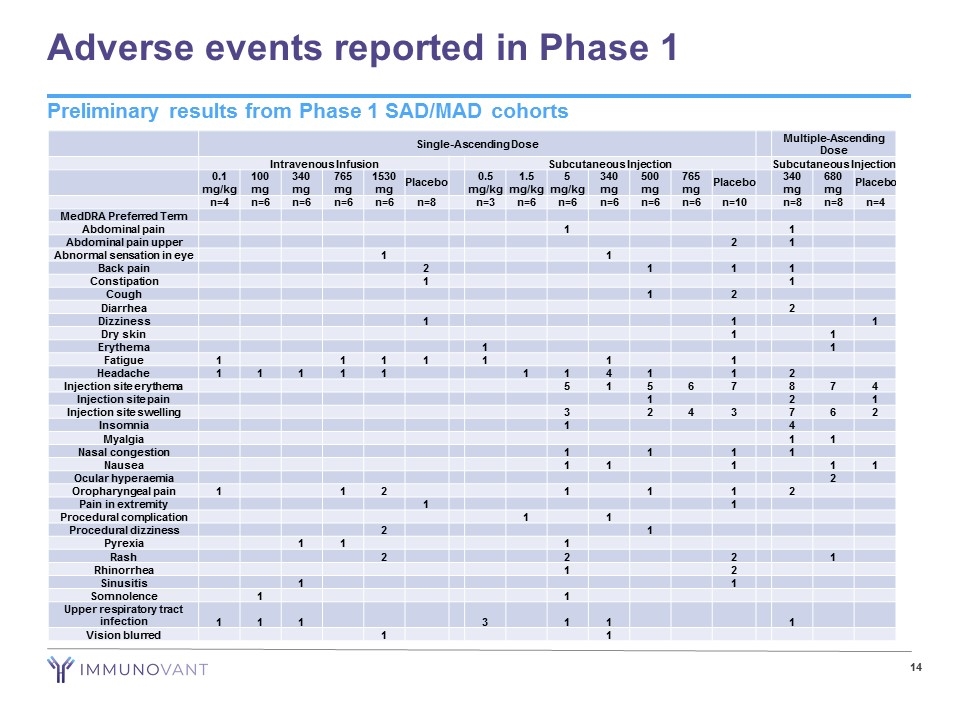
Adverse events reported in Phase 1 Single-Ascending Dose Multiple-Ascending Dose Intravenous Infusion Subcutaneous Injection Subcutaneous Injection 0.1 mg/kg 100 mg 340 mg 765 mg 1530 mg Placebo 0.5 mg/kg 1.5 mg/kg 5 mg/kg 340 mg 500 mg 765 mg Placebo 340 mg 680 mg Placebo n=4 n=6 n=6 n=6 n=6 n=8 n=3 n=6 n=6 n=6 n=6 n=6 n=10 n=8 n=8 n=4 MedDRA Preferred Term Abdominal pain 1 1 Abdominal pain upper 2 1 Abnormal sensation in eye 1 1 Back pain 2 1 1 1 Constipation 1 1 Cough 1 2 Diarrhea 2 Dizziness 1 1 1 Dry skin 1 1 Erythema 1 1 Fatigue 1 1 1 1 1 1 1 Headache 1 1 1 1 1 1 1 4 1 1 2 Injection site erythema 5 1 5 6 7 8 7 4 Injection site pain 1 2 1 Injection site swelling 3 2 4 3 7 6 2 Insomnia 1 4 Myalgia 1 1 Nasal congestion 1 1 1 1 Nausea 1 1 1 1 1 Ocular hyperaemia 2 Oropharyngeal pain 1 1 2 1 1 1 2 Pain in extremity 1 1 Procedural complication 1 1 Procedural dizziness 2 1 Pyrexia 1 1 1 Rash 2 2 2 1 Rhinorrhea 1 2 Sinusitis 1 1 Somnolence 1 1 Upper respiratory tract infection 1 1 1 3 1 1 1 Vision blurred 1 1 Preliminary results from Phase 1 SAD/MAD cohorts
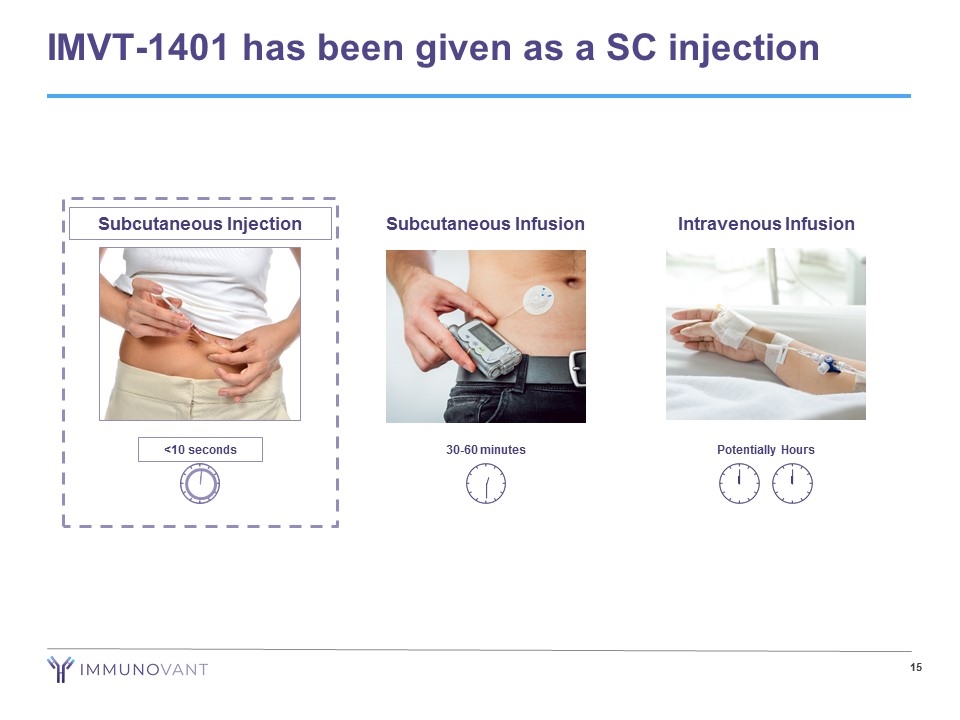
IMVT-1401 has been given as a SC injection Intravenous Infusion Potentially Hours Subcutaneous Injection <10 seconds Subcutaneous Infusion 30-60 minutes
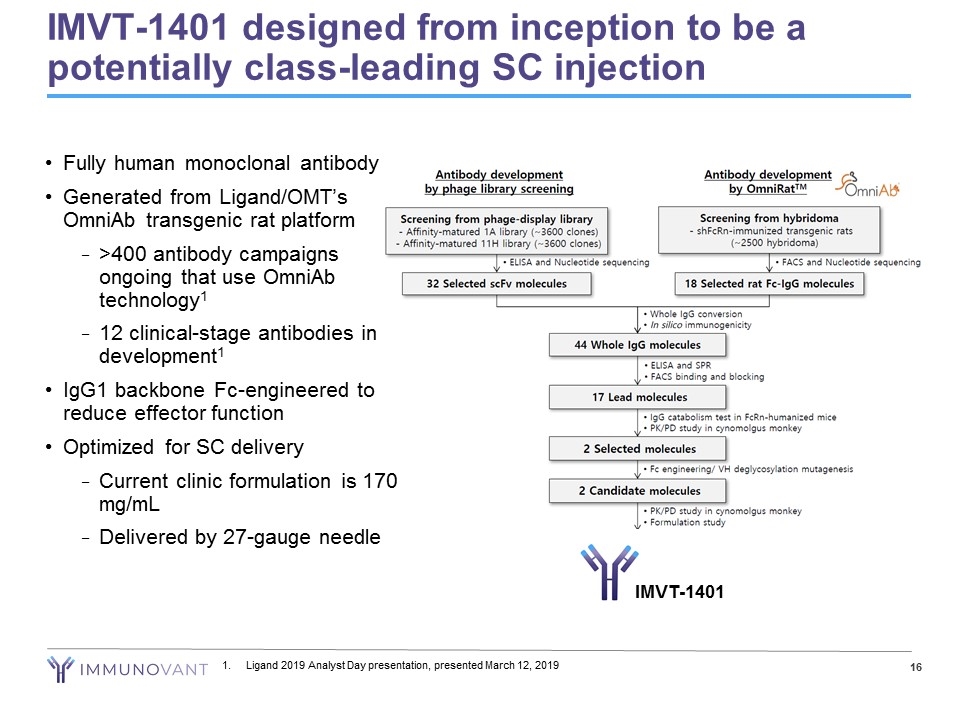
IMVT-1401 designed from inception to be a potentially class-leading SC injection Ligand 2019 Analyst Day presentation, presented March 12, 2019 IMVT-1401 Fully human monoclonal antibody Generated from Ligand/OMT’s OmniAb transgenic rat platform >400 antibody campaigns ongoing that use OmniAb technology1 12 clinical-stage antibodies in development1 IgG1 backbone Fc-engineered to reduce effector function Optimized for SC delivery Current clinic formulation is 170 mg/mL Delivered by 27-gauge needle

IMVT-1401 has the potential to deliver a class-leading profile IMVT-1401 attribute Potential patient benefit Clinically meaningful IgG reductions 680 mg SC weekly: 78% reduction after four doses 340 mg SC weekly: 63% reduction after four doses SC injection Fast and minimally invasive Simple dosing schedule No requirement for IV induction doses or lengthy SC infusions Provides option for at-home administration Fixed dosing, vs. weight-based, reduces potential for dose miscalculations Fully human antibody Low risk of immunogenicity Fc-engineered to reduce effector function Low potential for unintended immune responses

IMVT-1401 for Thyroid Eye Disease
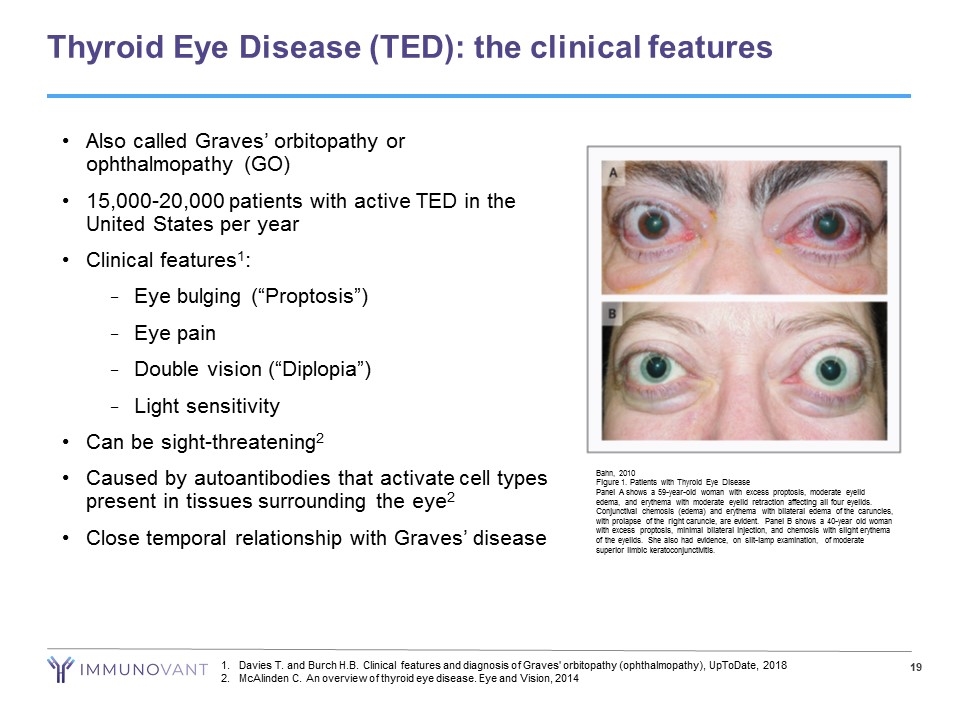
Thyroid Eye Disease (TED): the clinical features Bahn, 2010 Figure 1. Patients with Thyroid Eye Disease Panel A shows a 59-year-old woman with excess proptosis, moderate eyelid edema, and erythema with moderate eyelid retraction affecting all four eyelids. Conjunctival chemosis (edema) and erythema with bilateral edema of the caruncles, with prolapse of the right caruncle, are evident. Panel B shows a 40-year old woman with excess proptosis, minimal bilateral injection, and chemosis with slight erythema of the eyelids. She also had evidence, on slit-lamp examination, of moderate superior limbic keratoconjunctivitis. Also called Graves’ orbitopathy or ophthalmopathy (GO) 15,000-20,000 patients with active TED in the United States per year Clinical features1: Eye bulging (“Proptosis”) Eye pain Double vision (“Diplopia”) Light sensitivity Can be sight-threatening2 Caused by autoantibodies that activate cell types present in tissues surrounding the eye2 Close temporal relationship with Graves’ disease Davies T. and Burch H.B. Clinical features and diagnosis of Graves' orbitopathy (ophthalmopathy), UpToDate, 2018 McAlinden C. An overview of thyroid eye disease. Eye and Vision, 2014
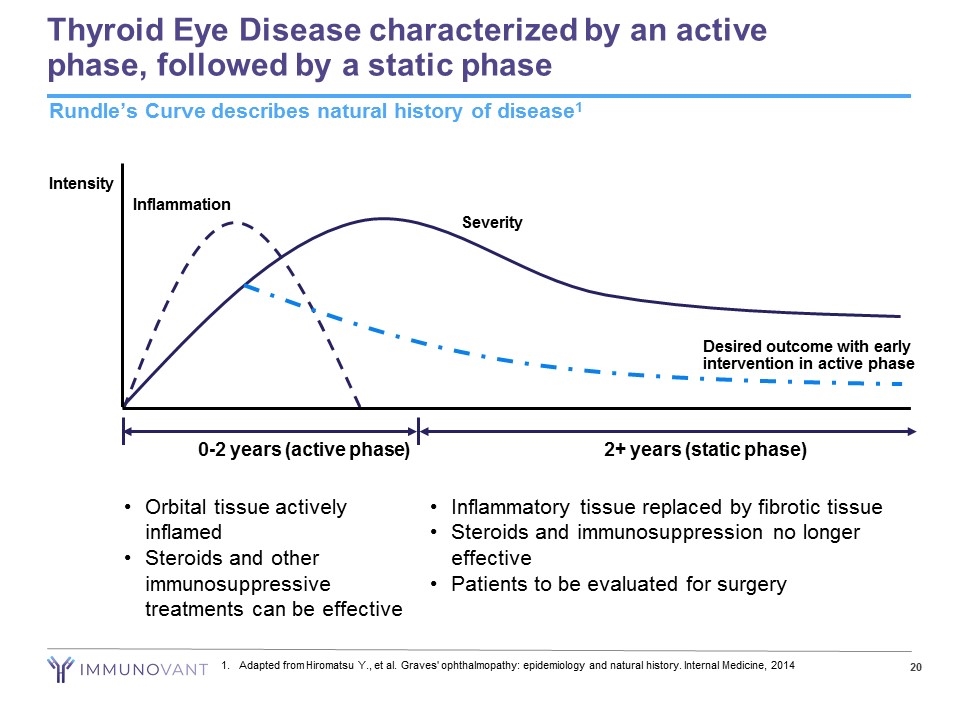
Thyroid Eye Disease characterized by an active phase, followed by a static phase Rundle’s Curve describes natural history of disease1 0-2 years (active phase) 2+ years (static phase) Intensity Severity Inflammation Orbital tissue actively inflamed Steroids and other immunosuppressive treatments can be effective Desired outcome with early intervention in active phase Inflammatory tissue replaced by fibrotic tissue Steroids and immunosuppression no longer effective Patients to be evaluated for surgery Adapted from Hiromatsu Y., et al. Graves' ophthalmopathy: epidemiology and natural history. Internal Medicine, 2014
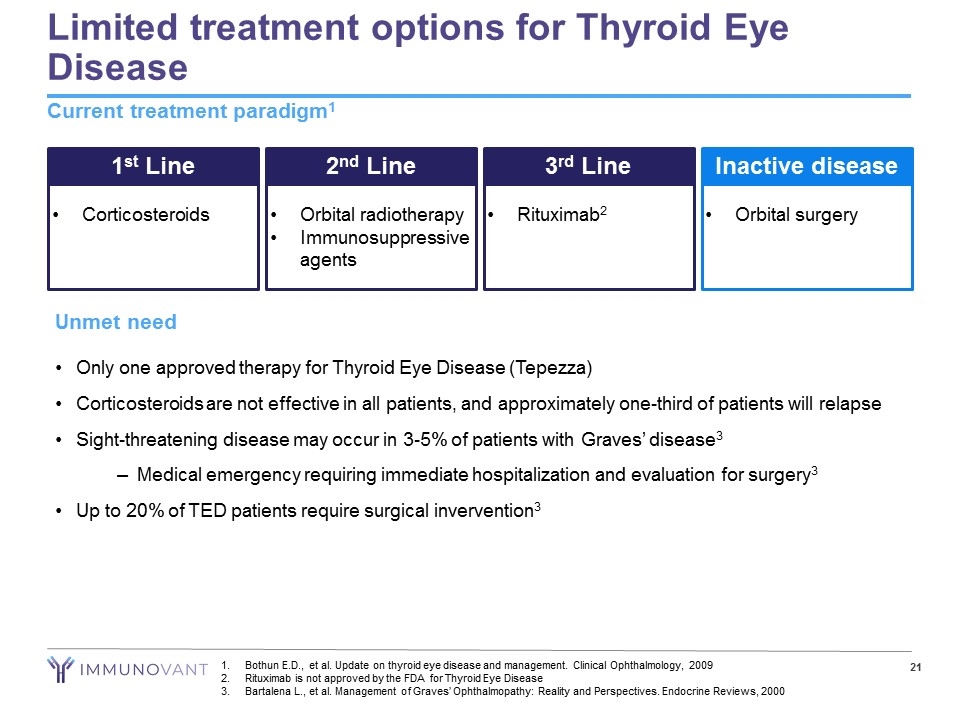
Limited treatment options for Thyroid Eye Disease Current treatment paradigm1 1st Line Corticosteroids 2nd Line Orbital radiotherapy Immunosuppressive agents 3rd Line Rituximab2 Inactive disease Orbital surgery Unmet need Only one approved therapy for Thyroid Eye Disease (Tepezza) Corticosteroids are not effective in all patients, and approximately one-third of patients will relapse Sight-threatening disease may occur in 3-5% of patients with Graves’ disease3 Medical emergency requiring immediate hospitalization and evaluation for surgery3 Up to 20% of TED patients require surgical invervention3 Bothun E.D., et al. Update on thyroid eye disease and management. Clinical Ophthalmology, 2009 Rituximab is not approved by the FDA for Thyroid Eye Disease Bartalena L., et al. Management of Graves’ Ophthalmopathy: Reality and Perspectives. Endocrine Reviews, 2000
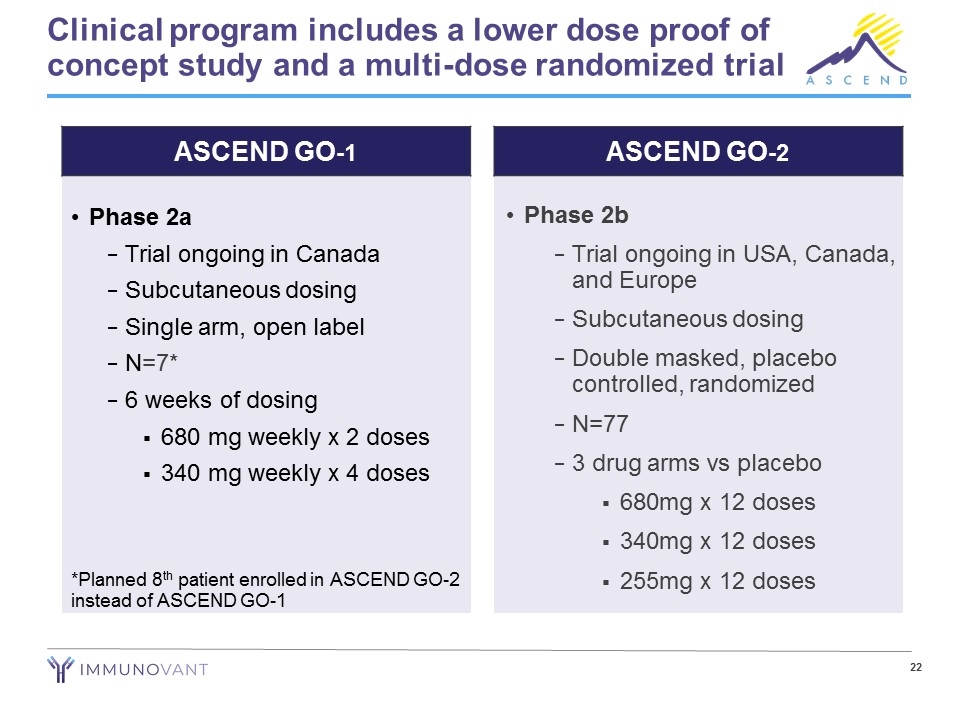
Clinical program includes a lower dose proof of concept study and a multi-dose randomized trial Phase 2a Trial ongoing in Canada Subcutaneous dosing Single arm, open label N=7* 6 weeks of dosing 680 mg weekly x 2 doses 340 mg weekly x 4 doses *Planned 8th patient enrolled in ASCEND GO-2 instead of ASCEND GO-1 Phase 2b Trial ongoing in USA, Canada, and Europe Subcutaneous dosing Double masked, placebo controlled, randomized N=77 3 drug arms vs placebo 680mg x 12 doses 340mg x 12 doses 255mg x 12 doses ASCEND GO-1 ASCEND GO-2
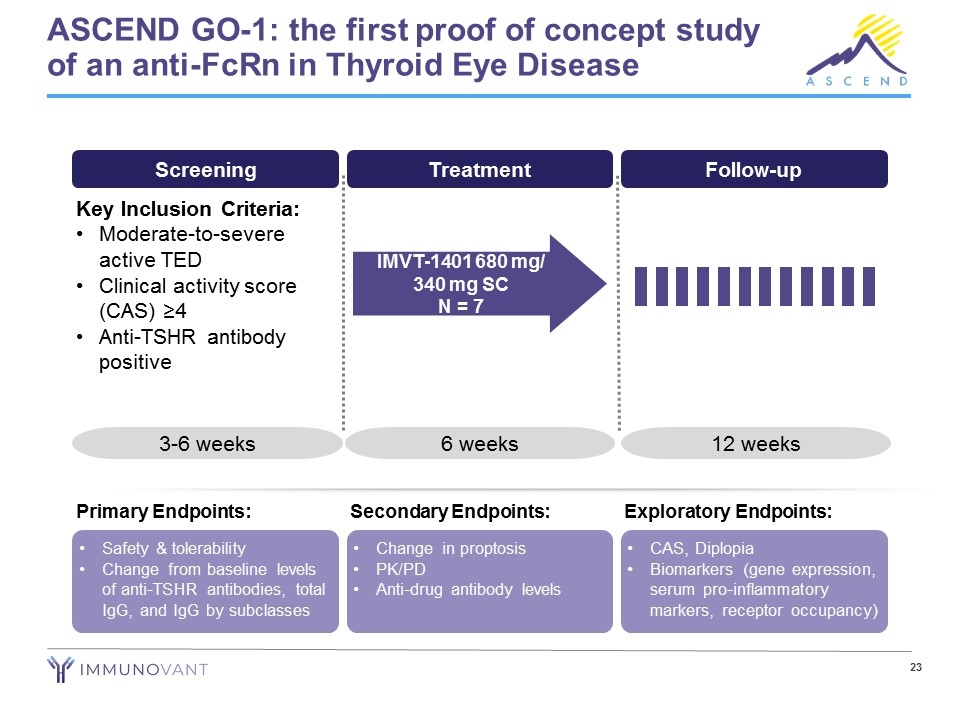
ASCEND GO-1: the first proof of concept study of an anti-FcRn in Thyroid Eye Disease Screening Treatment Follow-up Key Inclusion Criteria: Moderate-to-severe active TED Clinical activity score (CAS) ≥4 Anti-TSHR antibody positive 3-6 weeks 6 weeks 12 weeks IMVT-1401 680 mg/ 340 mg SC N = 7 Safety & tolerability Change from baseline levels of anti-TSHR antibodies, total IgG, and IgG by subclasses Primary Endpoints: Change in proptosis PK/PD Anti-drug antibody levels Secondary Endpoints: CAS, Diplopia Biomarkers (gene expression, serum pro-inflammatory markers, receptor occupancy) Exploratory Endpoints:
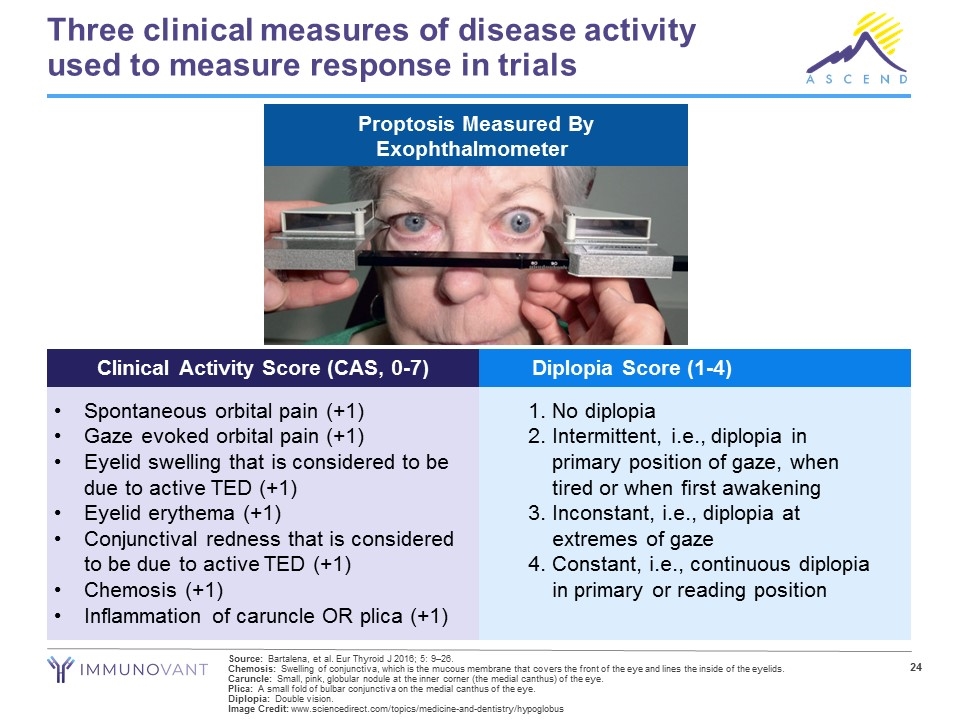
Three clinical measures of disease activity used to measure response in trials Source: Bartalena, et al. Eur Thyroid J 2016; 5: 9–26. Chemosis: Swelling of conjunctiva, which is the mucous membrane that covers the front of the eye and lines the inside of the eyelids. Caruncle: Small, pink, globular nodule at the inner corner (the medial canthus) of the eye. Plica: A small fold of bulbar conjunctiva on the medial canthus of the eye. Diplopia: Double vision. Image Credit: www.sciencedirect.com/topics/medicine-and-dentistry/hypoglobus Clinical Activity Score (CAS, 0-7) Diplopia Score (1-4) 1.No diplopia 2.Intermittent, i.e., diplopia in primary position of gaze, when tired or when first awakening 3.Inconstant, i.e., diplopia at extremes of gaze 4.Constant, i.e., continuous diplopia in primary or reading position Spontaneous orbital pain (+1) Gaze evoked orbital pain (+1) Eyelid swelling that is considered to be due to active TED (+1) Eyelid erythema (+1) Conjunctival redness that is considered to be due to active TED (+1) Chemosis (+1) Inflammation of caruncle OR plica (+1) Proptosis Measured By Exophthalmometer
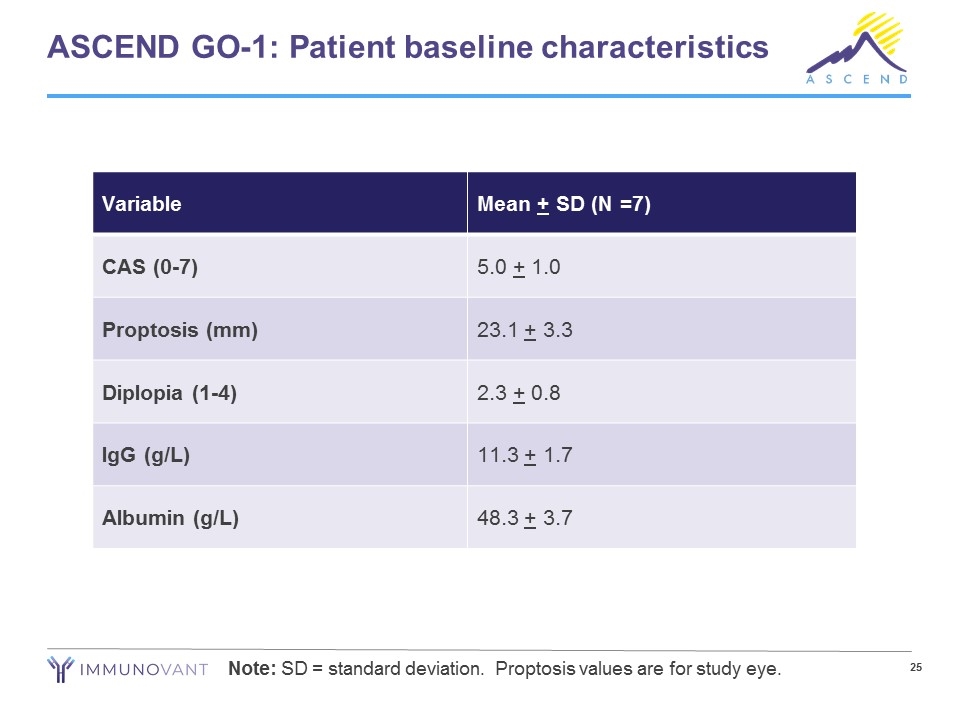
ASCEND GO-1: Patient baseline characteristics Note: SD = standard deviation. Proptosis values are for study eye. Variable Mean + SD (N =7) CAS (0-7) 5.0 + 1.0 Proptosis (mm) 23.1 + 3.3 Diplopia (1-4) 2.3 + 0.8 IgG (g/L) 11.3 + 1.7 Albumin (g/L) 48.3 + 3.7
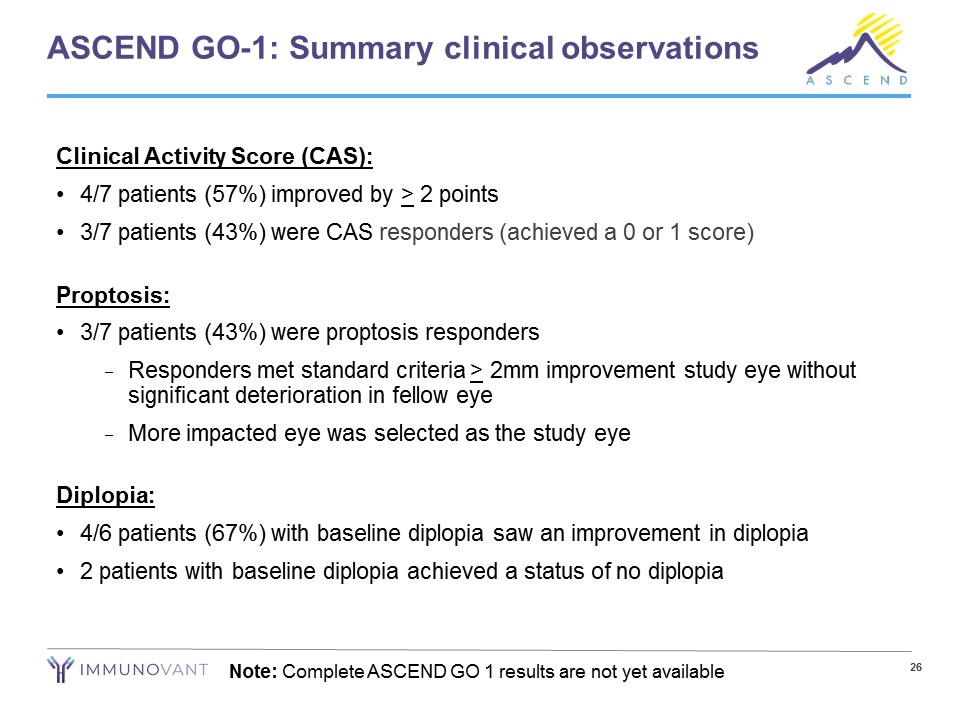
ASCEND GO-1: Summary clinical observations Clinical Activity Score (CAS): 4/7 patients (57%) improved by > 2 points 3/7 patients (43%) were CAS responders (achieved a 0 or 1 score) Proptosis: 3/7 patients (43%) were proptosis responders Responders met standard criteria > 2mm improvement study eye without significant deterioration in fellow eye More impacted eye was selected as the study eye Diplopia: 4/6 patients (67%) with baseline diplopia saw an improvement in diplopia 2 patients with baseline diplopia achieved a status of no diplopia Note: Complete ASCEND GO 1 results are not yet available
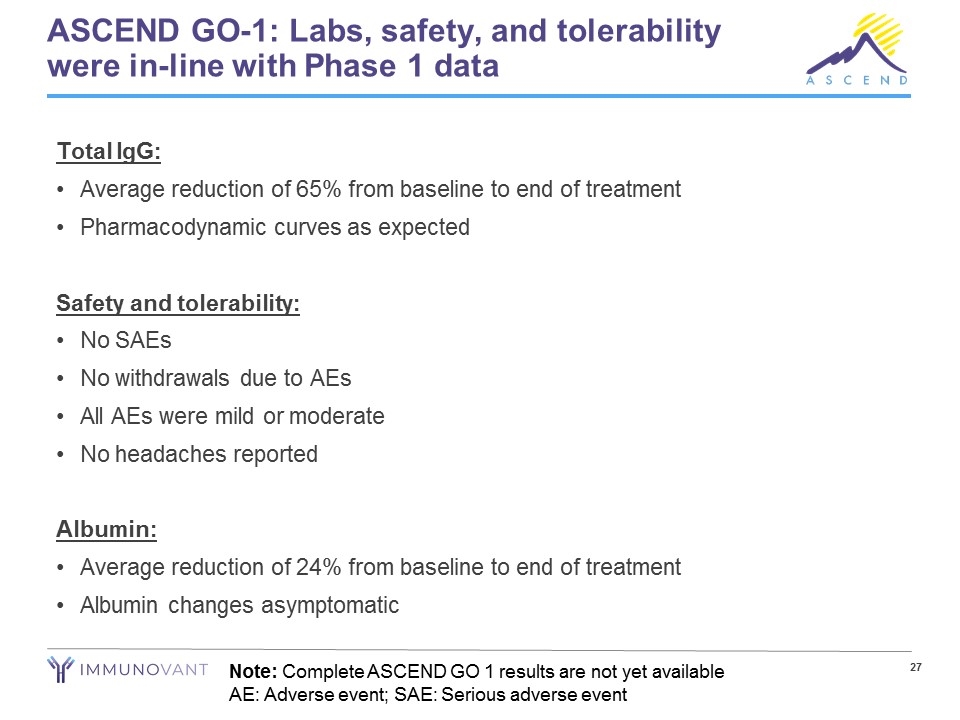
ASCEND GO-1: Labs, safety, and tolerability were in-line with Phase 1 data Total IgG: Average reduction of 65% from baseline to end of treatment Pharmacodynamic curves as expected Safety and tolerability: No SAEs No withdrawals due to AEs All AEs were mild or moderate No headaches reported Albumin: Average reduction of 24% from baseline to end of treatment Albumin changes asymptomatic Note: Complete ASCEND GO 1 results are not yet available AE: Adverse event; SAE: Serious adverse event
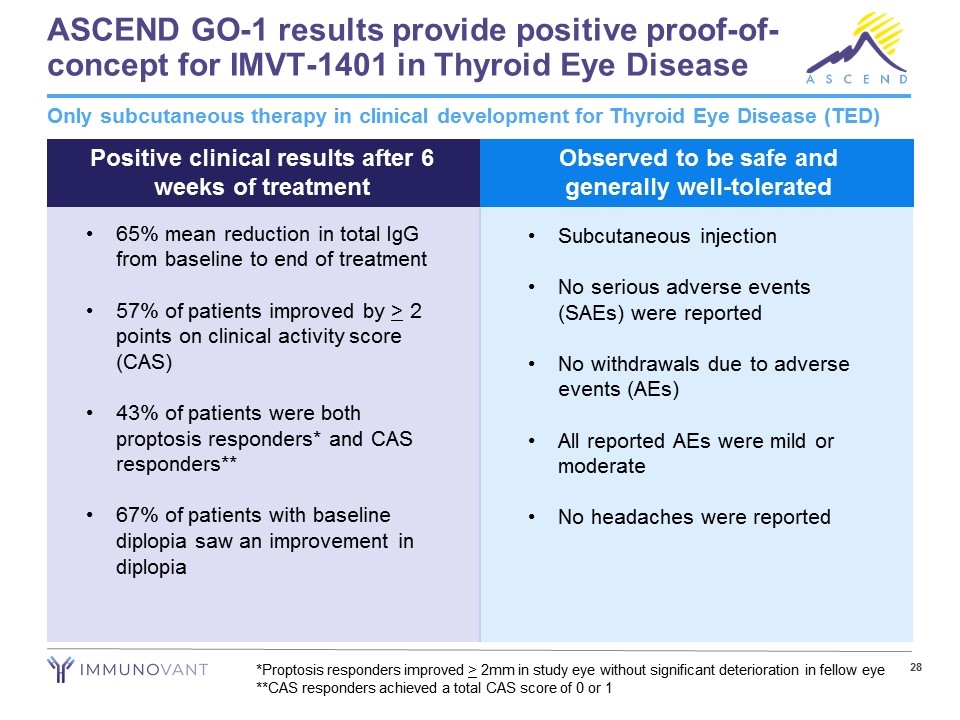
ASCEND GO-1 results provide positive proof-of-concept for IMVT-1401 in Thyroid Eye Disease Only subcutaneous therapy in clinical development for Thyroid Eye Disease (TED) Positive clinical results after 6 weeks of treatment Observed to be safe and generally well-tolerated Subcutaneous injection No serious adverse events (SAEs) were reported No withdrawals due to adverse events (AEs) All reported AEs were mild or moderate No headaches were reported 65% mean reduction in total IgG from baseline to end of treatment 57% of patients improved by > 2 points on clinical activity score (CAS) 43% of patients were both proptosis responders* and CAS responders** 67% of patients with baseline diplopia saw an improvement in diplopia *Proptosis responders improved > 2mm in study eye without significant deterioration in fellow eye **CAS responders achieved a total CAS score of 0 or 1
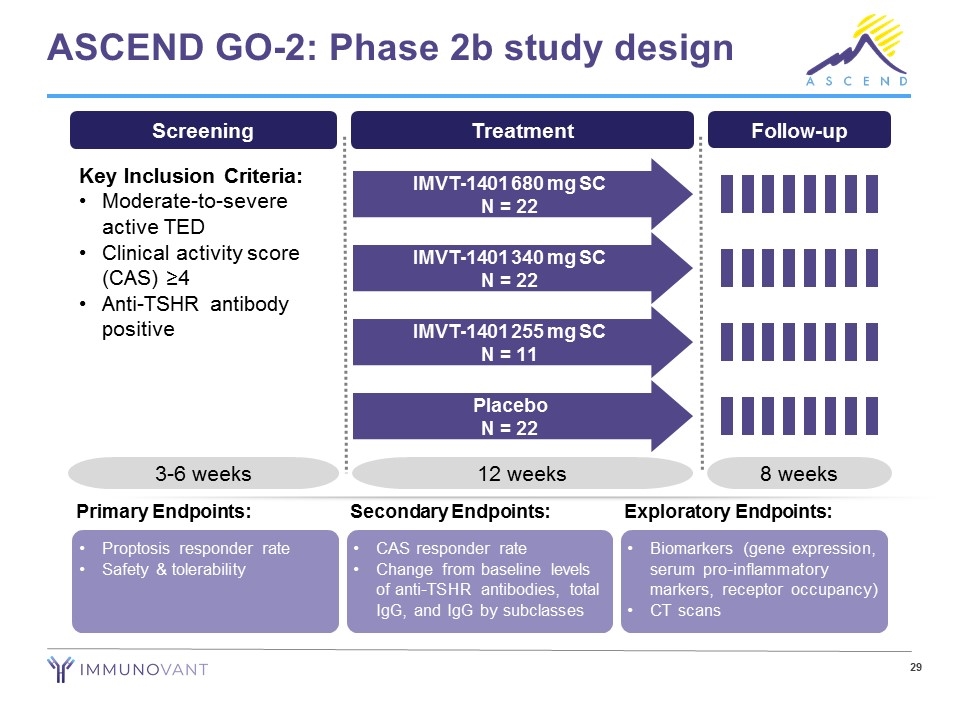
ASCEND GO-2: Phase 2b study design Screening Key Inclusion Criteria: Moderate-to-severe active TED Clinical activity score (CAS) ≥4 Anti-TSHR antibody positive 3-6 weeks Treatment 12 weeks IMVT-1401 680 mg SC N = 22 IMVT-1401 340 mg SC N = 22 IMVT-1401 255 mg SC N = 11 Placebo N = 22 Follow-up 8 weeks Proptosis responder rate Safety & tolerability Primary Endpoints: CAS responder rate Change from baseline levels of anti-TSHR antibodies, total IgG, and IgG by subclasses Secondary Endpoints: Biomarkers (gene expression, serum pro-inflammatory markers, receptor occupancy) CT scans Exploratory Endpoints:

IMVT-1401 for Myasthenia Gravis
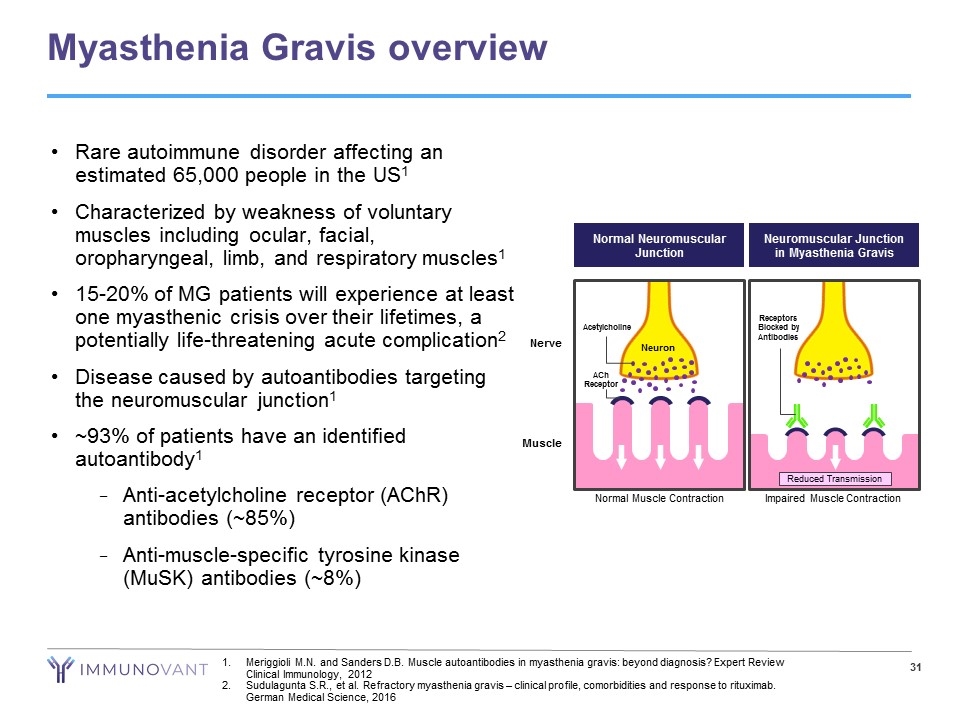
Myasthenia Gravis overview Meriggioli M.N. and Sanders D.B. Muscle autoantibodies in myasthenia gravis: beyond diagnosis? Expert Review Clinical Immunology, 2012 Sudulagunta S.R., et al. Refractory myasthenia gravis – clinical profile, comorbidities and response to rituximab. German Medical Science, 2016 Rare autoimmune disorder affecting an estimated 65,000 people in the US1 Characterized by weakness of voluntary muscles including ocular, facial, oropharyngeal, limb, and respiratory muscles1 15-20% of MG patients will experience at least one myasthenic crisis over their lifetimes, a potentially life-threatening acute complication2 Disease caused by autoantibodies targeting the neuromuscular junction1 ~93% of patients have an identified autoantibody1 Anti-acetylcholine receptor (AChR) antibodies (~85%) Anti-muscle-specific tyrosine kinase (MuSK) antibodies (~8%) Muscle Neuromuscular Junction in Myasthenia Gravis Reduced Transmission Impaired Muscle Contraction Receptors Blocked by Antibodies Neuron Nerve Acetylcholine ACh Receptor Normal Muscle Contraction Normal Neuromuscular Junction
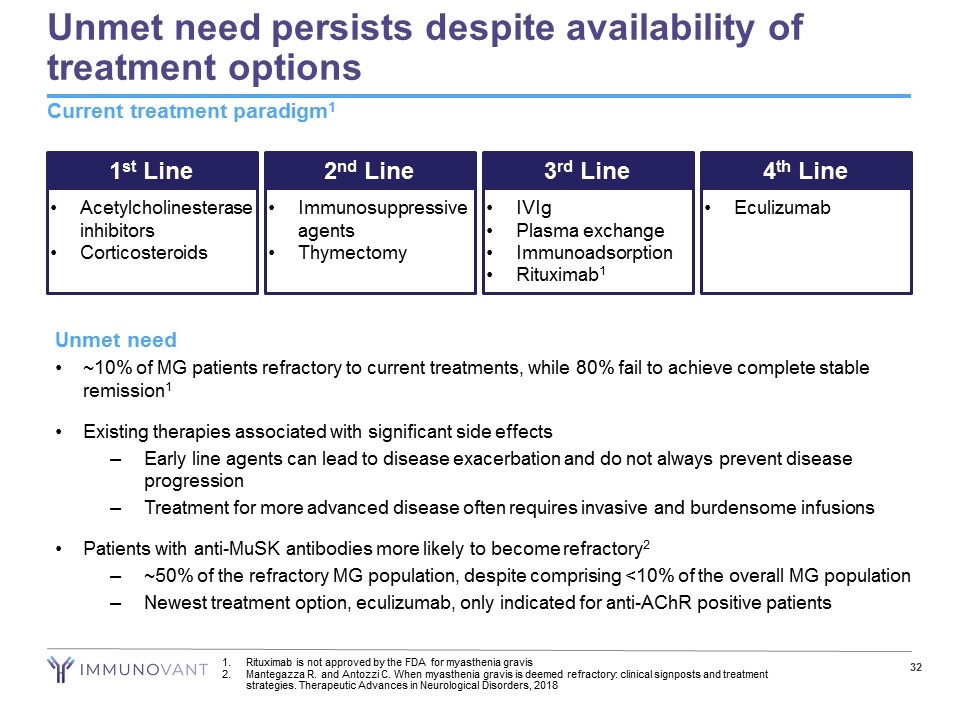
Unmet need persists despite availability of treatment options Current treatment paradigm1 1st Line Acetylcholinesterase inhibitors Corticosteroids 2nd Line Immunosuppressive agents Thymectomy 3rd Line IVIg Plasma exchange Immunoadsorption Rituximab1 4th Line Eculizumab Unmet need ~10% of MG patients refractory to current treatments, while 80% fail to achieve complete stable remission1 Existing therapies associated with significant side effects Early line agents can lead to disease exacerbation and do not always prevent disease progression Treatment for more advanced disease often requires invasive and burdensome infusions Patients with anti-MuSK antibodies more likely to become refractory2 ~50% of the refractory MG population, despite comprising <10% of the overall MG population Newest treatment option, eculizumab, only indicated for anti-AChR positive patients Rituximab is not approved by the FDA for myasthenia gravis Mantegazza R. and Antozzi C. When myasthenia gravis is deemed refractory: clinical signposts and treatment strategies. Therapeutic Advances in Neurological Disorders, 2018
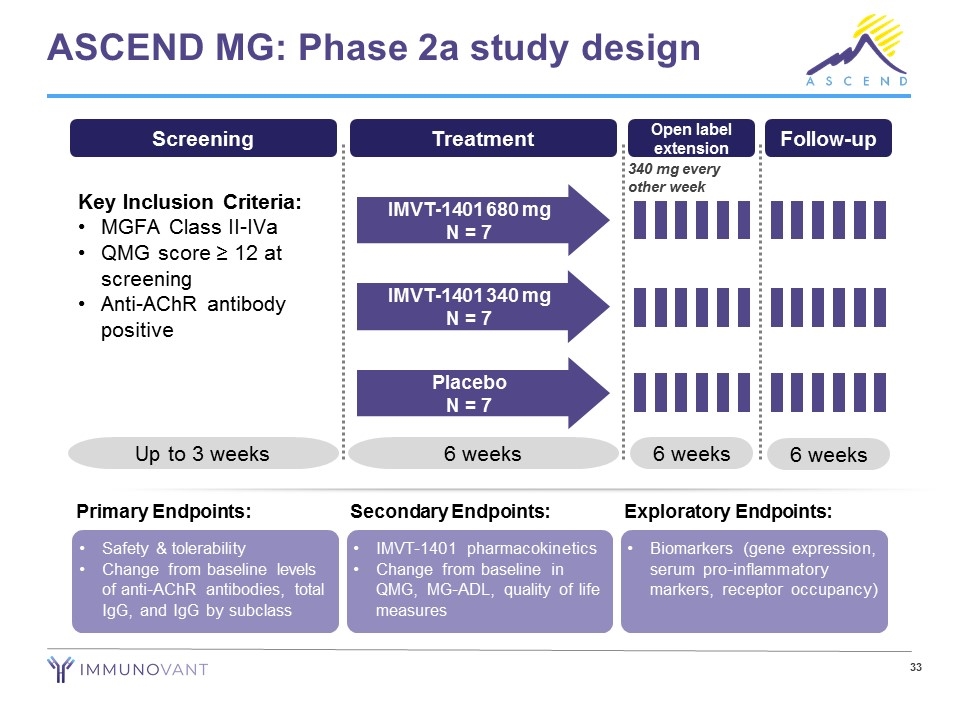
ASCEND MG: Phase 2a study design Safety & tolerability Change from baseline levels of anti-AChR antibodies, total IgG, and IgG by subclass Primary Endpoints: IMVT-1401 pharmacokinetics Change from baseline in QMG, MG-ADL, quality of life measures Secondary Endpoints: Biomarkers (gene expression, serum pro-inflammatory markers, receptor occupancy) Exploratory Endpoints: Screening Key Inclusion Criteria: MGFA Class II-IVa QMG score ≥ 12 at screening Anti-AChR antibody positive Up to 3 weeks Treatment 6 weeks IMVT-1401 680 mg N = 7 IMVT-1401 340 mg N = 7 Placebo N = 7 6 weeks Follow-up Open label extension 6 weeks 340 mg every other week

IMVT-1401 for Warm Autoimmune Hemolytic Anemia
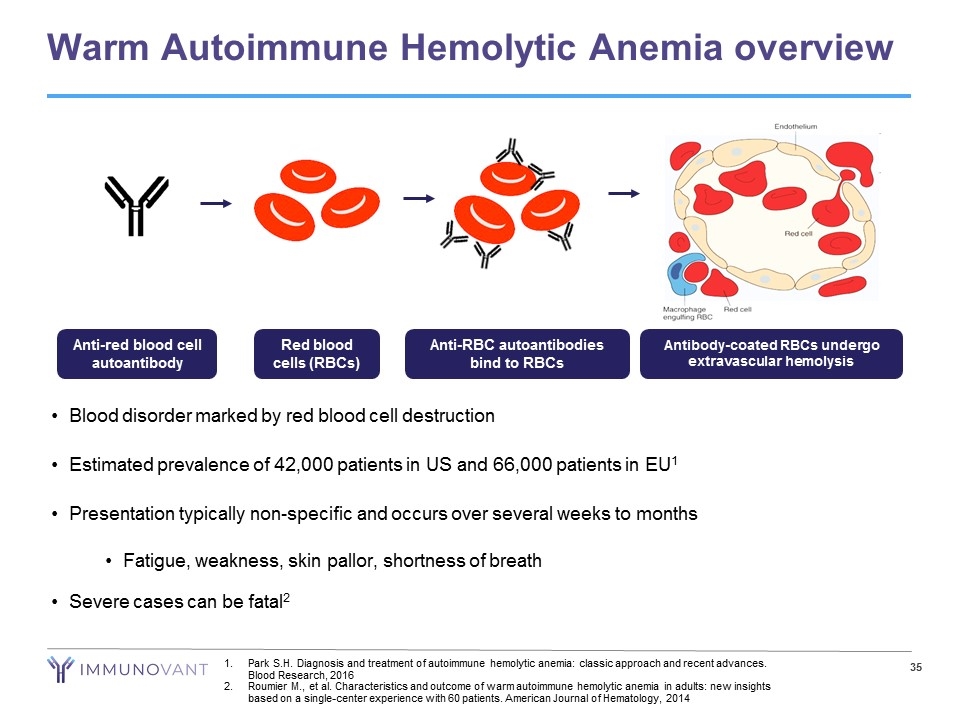
Warm Autoimmune Hemolytic Anemia overview Blood disorder marked by red blood cell destruction Estimated prevalence of 42,000 patients in US and 66,000 patients in EU1 Presentation typically non-specific and occurs over several weeks to months Fatigue, weakness, skin pallor, shortness of breath Severe cases can be fatal2 Anti-RBC autoantibodies bind to RBCs Anti-red blood cell autoantibody Red blood cells (RBCs) Antibody-coated RBCs undergo extravascular hemolysis Park S.H. Diagnosis and treatment of autoimmune hemolytic anemia: classic approach and recent advances. Blood Research, 2016 Roumier M., et al. Characteristics and outcome of warm autoimmune hemolytic anemia in adults: new insights based on a single-center experience with 60 patients. American Journal of Hematology, 2014
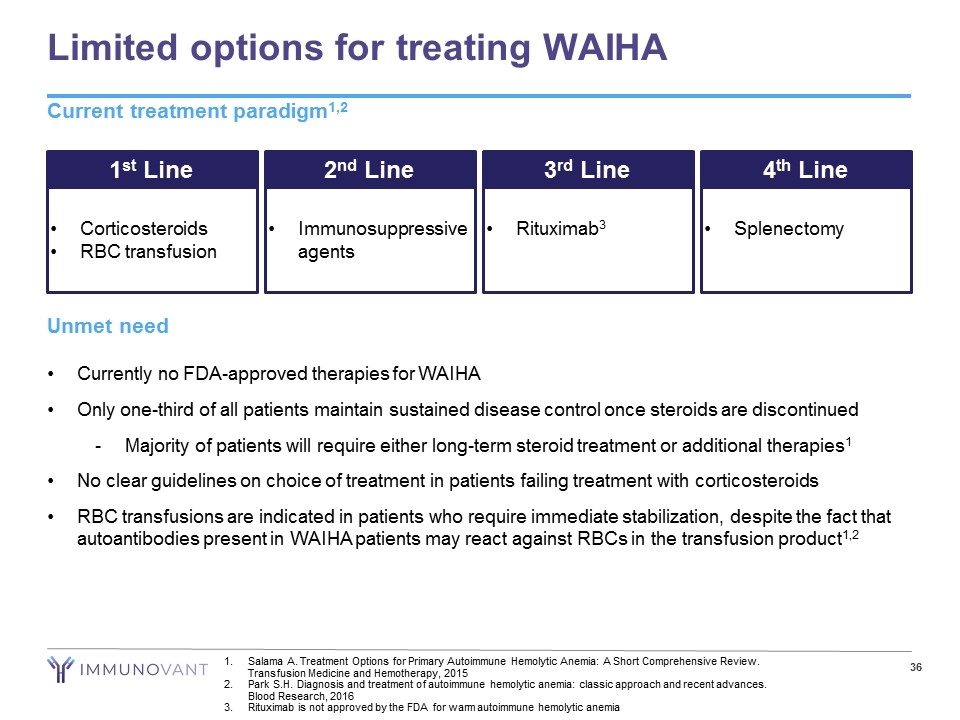
Limited options for treating WAIHA Current treatment paradigm1,2 1st Line Corticosteroids RBC transfusion 2nd Line Immunosuppressive agents 3rd Line Rituximab3 4th Line Splenectomy Unmet need Currently no FDA-approved therapies for WAIHA Only one-third of all patients maintain sustained disease control once steroids are discontinued Majority of patients will require either long-term steroid treatment or additional therapies1 No clear guidelines on choice of treatment in patients failing treatment with corticosteroids RBC transfusions are indicated in patients who require immediate stabilization, despite the fact that autoantibodies present in WAIHA patients may react against RBCs in the transfusion product1,2 Salama A. Treatment Options for Primary Autoimmune Hemolytic Anemia: A Short Comprehensive Review. Transfusion Medicine and Hemotherapy, 2015 Park S.H. Diagnosis and treatment of autoimmune hemolytic anemia: classic approach and recent advances. Blood Research, 2016 Rituximab is not approved by the FDA for warm autoimmune hemolytic anemia
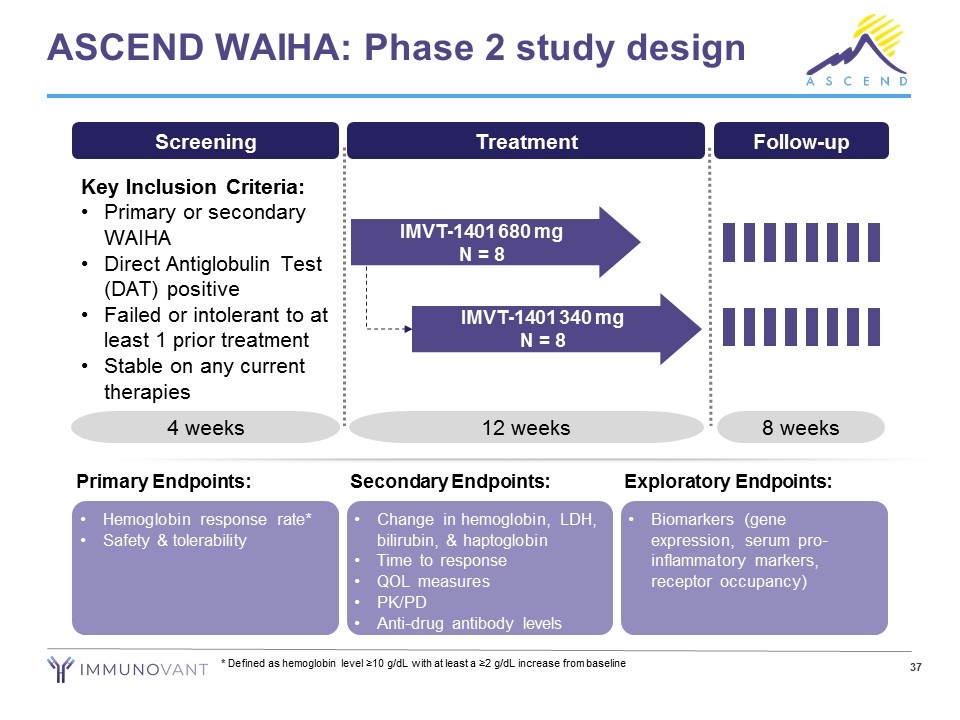
ASCEND WAIHA: Phase 2 study design Hemoglobin response rate* Safety & tolerability Primary Endpoints: Change in hemoglobin, LDH, bilirubin, & haptoglobin Time to response QOL measures PK/PD Anti-drug antibody levels Secondary Endpoints: Biomarkers (gene expression, serum pro-inflammatory markers, receptor occupancy) Exploratory Endpoints: * Defined as hemoglobin level ≥10 g/dL with at least a ≥2 g/dL increase from baseline Screening Key Inclusion Criteria: Primary or secondary WAIHA Direct Antiglobulin Test (DAT) positive Failed or intolerant to at least 1 prior treatment Stable on any current therapies 4 weeks Follow-up 8 weeks Treatment IMVT-1401 680 mg N = 8 IMVT-1401 340 mg N = 8 12 weeks
